High-Performance Waterborne Coatings
By Scott Sabreen, The Sabreen Group
 Decorative waterborne coatings provide exceptional appearance, functionality, value and are environmentally friendly. Waterborne coating is a fast-developing technology using water as the means to transfer the coating to the plastic surface and is becoming the new standard, replacing many of its solvent-based counterparts. Today’s waterborne chemistries can offer equal to or better cosmetic and physical performance properties than solventborne for many applications.
Decorative waterborne coatings provide exceptional appearance, functionality, value and are environmentally friendly. Waterborne coating is a fast-developing technology using water as the means to transfer the coating to the plastic surface and is becoming the new standard, replacing many of its solvent-based counterparts. Today’s waterborne chemistries can offer equal to or better cosmetic and physical performance properties than solventborne for many applications.
This is the first in a two-part series of waterborne coatings. This article will focus on basic single-component, air-dry technology. Part two will present alternative waterborne resin applications using acrylics, epoxies and urethanes.
Many of today’s paints and coatings contribute substantial quantities of VOCs (Volatile Organic Compounds) and HAPs (Hazardous Air Pollutants) which create poor air quality. The Clean Air Act of 1990 mandates significant reductions in the VOC and HAP levels generated by users of paints and coatings. Further, the California Air Resources Board (ARB) adopted a suggested control measure (SCM) for coatings in October 2005. The purpose of the SCM is to improve consistency and enforceability among air district rules and to achieve VOC emissions reductions beginning in 2009. The SCM serves as a model that each air district may adopt to meet the state implementation plan and California Clean Air Act requirements. In many cases this will require users to switch from solventborne coatings to alternative compliant coatings, such as waterborne.
High-performance waterborne coatings achieve ultrahigh decorative finish standards and are rapidly taking hold in many painting and coating market sectors. (For this article the words “paints” and “coatings” are used interchangeably.) Waterborne coatings are environmentally “green” and meet aggressive emissions regulations, whereby manufacturers have been able to change from solventborne paint systems in many applications. Waterborne coatings are less toxic, have low VOC levels and are less flammable. Their use will reduce air emissions and improve worker health and safety. Waterborne coatings are available in different curing mechanisms including a) evaporation, b) oven bake and c) UV-curable. In recent years, industries comprising the automotive, marine and architectural OEMs have dramatically increased their usage of pigmented and clearcoat (topcoat) waterborne coatings. Table 1 summarizes some of the many advantages for using waterborne coatings.
Basics of Waterborne Coating
In solventborne coatings, the fluidizing medium is an organic solvent or blend of organic solvents (MEK, MIBK, xylene, toluene, etc.). To be classified as a “waterborne” coating, water must be an important or major fluidizing medium. Most coatings that qualify as waterborne coatings also contain some organic co-solvents in the fluidizing media. These tend to be low-molecular weight polar ketones, alcohols and esters. Typical chemical composition is approximately 50-percent water, 45-percent solids and 5-percent co-solvents (can range from 2-20 percent of the fluidizing medium).
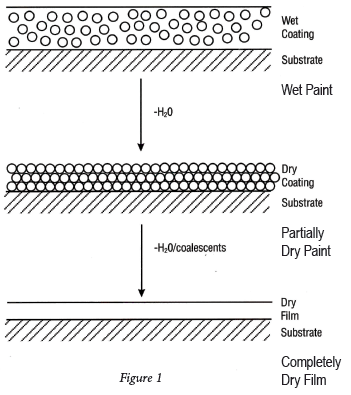 In order to understand the different methods of drying single-component waterborne coatings, it is necessary to understand the chemistry on how the water is removed from the coating itself. The water and co-solvents are removed from the surface of the paint coating via evaporation (physical drying or baking, without chemical cross-linking). The water below the surface migrates to the surface at a speed based upon the rate of diffusion of the coating. This process continues until all of the water has risen to the surface and is evaporated. The time in which the water is evaporated and the coating is dry is based upon the amount of water to be removed (i.e., thickness of coating and percentage of water in the paint) and the evaporation rate. The evaporation rate is dependent upon the vapor pressure difference between the water in the coating and the air being circulated over the surface of the part. The vapor pressure difference is a function of the humidity ratio (i.e., temperature and relative humidity of the circulating air) and to a smaller degree, the velocity of the air across the substrate.
In order to understand the different methods of drying single-component waterborne coatings, it is necessary to understand the chemistry on how the water is removed from the coating itself. The water and co-solvents are removed from the surface of the paint coating via evaporation (physical drying or baking, without chemical cross-linking). The water below the surface migrates to the surface at a speed based upon the rate of diffusion of the coating. This process continues until all of the water has risen to the surface and is evaporated. The time in which the water is evaporated and the coating is dry is based upon the amount of water to be removed (i.e., thickness of coating and percentage of water in the paint) and the evaporation rate. The evaporation rate is dependent upon the vapor pressure difference between the water in the coating and the air being circulated over the surface of the part. The vapor pressure difference is a function of the humidity ratio (i.e., temperature and relative humidity of the circulating air) and to a smaller degree, the velocity of the air across the substrate.
Air-dry and oven-bake waterborne chemistries cure in the sequence shown in Figure 1. The organic co-solvent evaporates, water evaporates and the resin particles coalesce to form a continuous coating film.
Process Application – Conventional application processes can be used with waterborne coatings, including all of the various spray methods and dip coating. This gives waterborne processes an advantage over high-solid paints which cannot be dip coated due to their higher viscosities. Spraying methods include traditional air atomization, HVLP, airless and air-assisted airless. Paint guns can be cleaned with water or water-based solutions rather than paint thinner or acetone.
Drying Considerations – The curing time for waterborne coatings is much longer than their solventborne counterparts. Adequate flash-off time between the spray booth and oven is necessary when force drying or baking the coating, otherwise solvent popping may result. It also is necessary to ensure that the surface temperature of the part is greater than the dew point to prevent condensation from forming. While this is generally not a problem during warmer months, it can be a concern during colder months. Warmer weather when the humidity is high can extend flash-off and dry times. If the humidity is high, the water vapor released during drying has no place to go, and the film will not cure. By providing moderate air flow and increased temperature, a continuous supply of fresh air can be provided to the coating to give the air more capacity to hold moisture. All water must be removed from the coating before parts are exposed to freezing temperatures. Failure to do so may result in a loss of adhesion, as the remaining water will expand upon freezing.
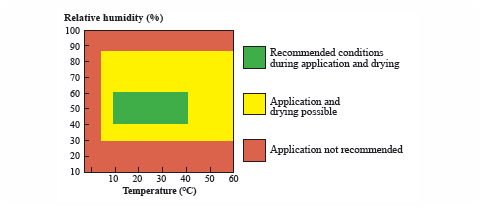
During the application of waterborne coatings, it is important to have tightly controlled temperature, humidity and ventilation (see Figure 2, page 42). Ideal conditions (area highlighted in green) for optimal “spraying” operations are 40 to 60 percent relative humidity and temperature between 10-25ºC. For “drying”, the optimal conditions are relative humidity less than 60 percent, temperature between 10-40ºC. Excellent ventilation is equally important. The larger yellow highlighted area represents the extreme limits for humidity and temperature in which spraying and drying are possible but less than optimal. Each product application is unique so that the ideal ambient conditions must be determined and tightly controlled.
Preventing Paint Failures – Many waterborne and solventborne coating failures result from inadequate surface preparation. These include both cosmetic defects and performance defects. The degree of cleanliness and higher surface energy required for waterborne coatings is greater than that necessary for most solventborne coatings due to the low solvent content. Solventborne coatings are more forgiving of residue because the solvents contained in the coatings may dissolve some surface oils and contaminates. Even though waterborne coatings typically contain “co-solvents,” they still contain much less organic solvent than solventborne coatings.
A major contributing factor to these problems, particularly robust adhesion, is that many plastics are chemically inert, nonporous with low surface energy. That is, most plastics are hydrophobic and are not naturally wettable. As a general rule, acceptable bonding adhesion is achieved when the surface energy of the substrate (measured in dynes/cm) is approximately 8-10 dynes/cm greater than the surface tension of the liquid. In this situation, the liquid is said to “wet out” or adhere to the surface. Surface pretreatments are used to increase surface energy and improve the wetting and adhesive properties of polymer materials. A variety of gas-phase surface oxidation pretreatment processes are used including low pressure cold gas plasma (RF or microwave), electrical (corona discharge), and flame plasma. Each method is application-specific and possesses unique advantages and potential limitations. The use of chemical primers (chlorinated polyolefins) also can be used alone or in conjunction with a gas-phase pretreatment.
Considerations
As with most applications and technologies there are advantages, potential limitations and process challenges also exist. First, consider whether waterborne coating is the best choice relative to conventional solventborne coatings, high-solids solvent coatings or another alternative. Within the category of waterborne coatings there are several types of “binders” to consider including epoxies, polyesters and urethanes. Binders are chosen based on the physical and chemical properties required of the finished film. Single-component and two-component chemistries are available if cross-linking is required. Waterborne coating processes are much less forgiving than solventborne and they are very sensitive to workplace environment fluctuations.
A common mispractice of curing waterborne coatings when issues arise is to simply increase temperature and airflow velocity. However, re-circulating “humid” air during curing actually decreases the vapor pressure differential and increases cure time. Surface pretreatment, whether chemical or oxidative, is almost always needed to prevent application flow (sagging) and achieve robust dry film coating adhesion.
In conclusion, one of the most compelling reasons for using waterborne coatings is their environmentally friendly low to zero-VOC content. Waterborne coating chemistries can offer equal to or better cosmetic and physical performance properties than solventborne for many applications, including clear topcoat applications. Due to regulatory pressures, solventborne coatings are being phased out whenever possible. Significant research and development is rapidly increasing to introduce new and improved waterborne coatings.
Acknowledgements
Waterborne Coatings Design Considerations – American Coatings Conference, Ivan Tyre, Alberdingk Boley & Tim December, BASF
Marine and Protective Coatings, Jotun Group
Industrial Painting, Norman Roobol
Surface Wetting and Pretreatment Methods, Plastics Decorating Magazine, Scott R. Sabreen, The Sabreen Group, Inc.